Battery Definitions, Terms & Terminology
Battery technology is becoming more important in the electrical & electronics industry, with this the terms and definitions are becoming more widely used.
Home » Electronic components » this page
Battery Technology Includes:
Battery technology overview
Battery definitions & terms
Battery capacity & life
Batteries / cells in series & parallel
Zinc carbon
Alkaline cells
Zinc air cells
Lithium primary battery
NiCad
NiMH
Li-ion
Lead acid
Battery leakage cleaning, cures
With battery technology becoming an increasingly important element of the electronics and electrical industries, the terminology and definitions are becoming more widely used.
When discussing batteries and cell technology it is often useful to be able to utilise the correct battery terms and terminology and definitions.
Accordingly a list of some of the more commonly used battery terminology and definitions have been compiled below:

With battery technology being used ever more widely, not only for small electronic gadgets as well as mobile phones and the like as well as within electric cars and many other pieces of electrical and electronic equipment, these terms are being used increasingly in many areas of everyday life.
Battery definitions, terms, & terminology
There are many different terms and sets of terminology used with batteries and cells regardless of whether they are rechargeable batteries or not.
It is often useful to have a basic understanding of the various terms even for buying batteries and cells in a shop, or for a more technical understanding when they are being used in electrical or electronic circuit designs.
The commonly used battery terms and terminology are defined in the list below:
Anode: The definition for the anode is the electrode at which an oxidation reaction occurs. This means that the anode electrode is a supplier of electrons. However the electron flow reverses between charge and discharge activities. As a result, the positive electrode is the anode during charging and the negative electrode is the anode during discharging.
In order to prevent confusion, the anode is normally defined for its activity during the discharge cycle. In this way the term anode is used for the negative electrode in a cell or battery.
- Battery: A battery is the generic name for a unit that creates electrical energy from stored chemical energy. Strictly it consists of two or more cells connected in an appropriate series / parallel arrangement to provide the required operating voltage and capacity to meet its operating requirements. The term battery is also frequently used to refer to a unit consisting of a single cell, especially when it contains battery management circuitry.
Cathode: The definition of a cathode is the electrode in a battery or other system at which a reduction reaction occurs. The electrode takes up electrons from an external circuit. Accordingly, the, the negative electrode of the battery or cell is the cathode during charging and the positive electrode is the cathode during discharging.
To prevent confusion the cathode is normally specified for the discharge cycle. As a result, the name cathode is commonly used for the positive electrode of the cell or battery.
- Capacity: The capacity of a battery or cell is defined as the amount of energy that it can deliver in a single discharge. Battery capacity is normally specified in amp-hours for larger batteries and mill-amp-hours for smaller batteries.
The capacity may be quoted on the battery itself and also in the specification. Many rechargeable cells and batteries have the charge capacity stated on the outer casing, whereas it is normally necessary to look int he manufacturers specifications for non-rechargeables.
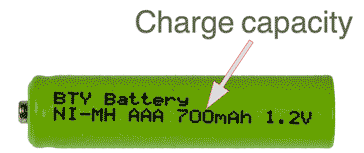
Charge capacity shown on the case of a rechargeable cell - in this case an NiMH cell The battery capacity may also be quoted in terms of watt-hours as this is a measure of the power it can supply, and this measure is the charge capacity in terms of amp-hours or milliamp hours times the voltage.
- Cell: The definition of the cell is the basic electrochemical unit that is used to create electrical energy from stored chemical energy or to store electrical energy in the form of chemical energy. A basic cell consists of two electrodes with an electrolyte between them.
Charge capacity: The capacity of a battery is often referred to as the charge capacity as this is the amount of charge the battery can hold. See "Capacity."
- Charge rate or C-rate : The definition of the charge rate or C-rate of a battery or cell is the charge or discharge current in Amperes as a proportion of the rated capacity in Ah. For example, in the case of a 500 mAh battery, a C/2 rate is 250 mA and a 2C rate would be1 A.
- Constant-Current Charge: This refers to a charging process where the level of current is maintained at a constant level regardless of the voltage of the battery or cell.
- Constant-Voltage Charge: - This definition refers to a charging process in which the voltage applied to a battery is held at a constant value over the charge cycle regardless of the current drawn.
Cycle Life: The capacity of a rechargeable cell or battery changes over its life. The definition of the battery life or cycle life of a battery is number of cycles that a cell or battery can be charged and discharged under specific conditions, before the available capacity falls to a specific performance criteria - normally 80% of the rated capacity.
The cycle life of batteries is gaining even more in importance. Mobile phones are often replaced when their batteries come to the end of their life, and for electric cars the battery life time is of great importance as replacement of the batteries is a very expensive operation.
NiMH batteries typically have a cycle life of 500 cycles, NiCd batteries can have a cycle life of over 1,000 cycles and for NiMH cells it is less at around 500 cycles. Lithium -ion cells currently have cycle life times of around 300 cycles, although with development this is improving. The cycle life of a cell or battery is greatly influenced by the type depth of the cycle and the method of recharging. Improper charge cycle cut-off, particularly if the cell is over-charged or reverse charged significantly reduces the cycle life.
- Cut-off voltage: As a battery or cell is discharged it has a voltage curve that it follows - the voltage generally falling over the discharge cycle. The definition for a cell or battery of the cut-off voltage cell or battery is the voltage at which the discharge is terminated by any battery management system. This point may also be referred to as the End-of-Discharge voltage.
- Deep Cycle: A charge discharge cycle in which the discharge is continued until the battery is fully discharged. This is normally take to be the point at which it reaches its cut-off voltage, typically 80% of discharge.
- Electrode: The electrodes are the basic elements within an electrochemical cell. There are two in each cell: one positive and one negative electrode. The cell voltage is determined by the voltage difference between the positive and the negative electrode.
- Electrolyte: The definition of the electrolyte within a battery is that it is the medium that provides the conduction of ions between the positive and negative electrodes of a cell.
- Energy Density: The volumetric energy storage density of a battery, expressed in Watt-hours per litre (Wh/l).
- Power Density: The volumetric power density of a battery, expressed in Watts per litre (W/l).
Primary cell: A primary cell is one that is not rechargeable. Zinc carbon, alkaline, silver and many other forms of cell are not rechargeable and these are all termed primary cells.
- Rated Capacity: The capacity of a battery is expressed in Ampere-hours, Ah and it is the total charge that can be obtained from a fully charged battery under specified discharge conditions. This is effectively a more exact term for the capacity of the cell or battery.
- Self-Discharge: It is found that batteries and cells will lose their charge over a period of time, and need re-charging. This self-discharge is normal, but various according to a number of variables including the technology used and the conditions. Self-discharge is defined as the recoverable loss of capacity of a cell or battery. The figure is normally expressed in a percentage of the rated capacity lost per month and at a given temperature. The self-discharge rate of a battery or cell is very dependent upon the temperature.
Secondary cell: A secondary cell is one that can be recharged. Nickel Cadmium (not liked these days because of their environmental effects) Nickel Metal Hydride, Lithium Ion, Lead Acid and others are all rechargeable and are therefore referred to as secondary cells and battery technologies.
- Separator: This battery terminology is used to define the membrane that is required within a cell to prevent the anode and cathode shorting together. With cells being made more compact, the space between the anode and cathode becomes much smaller and as a result the two electrodes could short together causing a catastrophic and possibly explosive reaction. The separator is an ion-permeable, electronically non-conductive material or spacer that is placed between the anode and cathode.
- Specific Energy: The gravimetric energy storage density of a battery, expressed in Watt-hours per kilogram (Wh/kg).
- Specific Power: The specific power for a battery is the gravimetric power density expressed in Watts per kilogram (W/kg).
- Trickle charge: This terms refers to a form of low level charging where a cell is either continuously or intermittently connected to a constant-current supply that maintains the cell in fully charged condition. Current levels may be around 0.1C or less dependent upon the cell technology.
The list of battery terms and definitions above lists the more commonly used terms and definitions. Others will be used from time to time, but they are not in such common use.
 Written by Ian Poole .
Written by Ian Poole .
Experienced electronics engineer and author.
More Electronic Components:
Batteries
Capacitors
Connectors
ADC
DAC
Diodes
FET
Inductors
Memory types
Phototransistor
Quartz crystals
Relays
Resistors
RF connectors
Switches
Surface mount technology
Thyristor
Transformers
Transistor
Unijunction
Valves / Tubes
Return to Components menu . . .