Alkaline Manganese Dioxide Battery / Cell Technology
The alkaline manganese dioxide or 'alkaline' cells & batteries use a zinc anode, manganese dioxide cathode & alkaline electrolyte to give a much higher capacity than standard zinc carbon cells.
Home » Electronic components » this page
Battery Technology Includes:
Battery technology overview
Battery definitions & terms
Battery capacity & life
Batteries / cells in series & parallel
Zinc carbon
Alkaline cells
Zinc air cells
Lithium primary battery
NiCad
NiMH
Li-ion
Lead acid
Battery leakage cleaning, cures
Alkaline manganese dioxide cells or as they are often marketed, alkaline batteries and cells are now the major form of primary or non-rechargeable cell that are sold these days.
The alkaline cells have taken over from the older zinc carbon batteries and cells that were prevalent until the 1990s or thereabouts.

The alkaline manganese dioxide technology enables these cells and batteries to provide a much higher level of charge - they last much longer than their zinc carbon counterparts.
In view of their chemistry, these cells may also be referred to as Zn-MnO2 as this describes the chemicals used within the cell.
In view of their improved charge capacity, alkaline cells are used in many toys, torches and many other electronic circuit designs and general devices where lithium ion batteries might not be so suitable, or cost may be an issue.
Names like Duracell, and Energizer, etc have now dominated the market, although many other cheaper brands are also available. In fact nowadays it is not easy to find the older batteries such as the cells using zinc carbon battery technology.
How alkaline cells were developed - history
Traditionally electrochemical cells and batteries have used an acid as the electrolyte. The first step towards the development of the alkaline manganese dioxide cells of today was taken when the first cells using an alkali as the electrolyte was developed by Waldemar Jungner in 1899.
Ernst Waldemar Jungner was a Swedish inventor and engineer who invented a number of types of battery and cell including the nickel iron battery, NiFe, and also the Nickel cadmium rechargeable cell. He lived between 1869 and 1924.
Two years later and working independently, Thomas Edison also developed a cell technology based around an alkaline electrolyte.
Although the foundations for the modern alkaline battery had been set in place as early as the turn of the 1800 / 1900 centuries it took many years before the foundations of the modern alkaline manganese dioxide cell were set in place.
The credit for this goes to a Canadian engineer named Lewis Urry who undertook work independently in the 1950s. Then when he worked for Union Carbide's battery division made Eveready, work progressed more rapidly and along with P A Marsal, they filed US patent 2 960 558 for the alkaline battery based around alkaline manganese dioxide technology. This was granted in 1960.
One of the drawbacks of cells of the time was that the zinc electrode, also used in zinc carbon cells required a thin surface film of mercury. As mercury became less acceptable for use, improvements in the purity of the zinc used enabled the mercury content of batteries to be removed.
The success of the alkaline cell using manganese dioxide and zinc for the electrodes rose dramatically as they promised far higher charge capacities and lasted much longer. The issues with leakage that had dogged the older zinc carbon cells were also significantly reduced.
Accordingly in the 1990s and beyond the alkaline cells took off and became the dominant form of primary, non-rechargeable cells used in a host of items of electrical and electronic equipment.

Basic alkaline manganese diode cell characteristics
The alkaline manganese dioxide cells provide a high level of performance and in some instances they may out-perform lithium ion batteries and cells.
| Common Sizes for Alkaline battery Cells | ||
|---|---|---|
| Attribute | Details | |
| Nominal cell voltage | 1.5V | |
| Self discharge rate | Typically less than 0.3% per month | |
| Battery / cell lifetime | Typically 5 - 10 years | |
Alkaline batteries come in a variety of different sizes ranging from AAA to AA, C, D, PP3 / 6LR61 and others.
In view of their size, and the internal structures used, the AAA and AA sizes are generally most suited for low-drain applications although being larger, the AA size can also be used for some high-drain applications.
The C, and D cell sizes are are suited for high-drain applications although their higher charge capacity means they are often used for medium drain applications where infrequency changes are required.
There are other sizes, but these are much less widely used. These include micro alkaline button cells, coin cells, AAAA and the like.
Of all the alkaline manganese dioxide cells, the AA size is the most widely used alkaline battery cell size, while the AAA size is close behind.
Size C, D and 9 V are used for specific applications which have steady demand. However, other sizes such as micro alkaline coin cells and button cells are used in few industrial and medical applications.
| Common Sizes for Alkaline battery Cells | ||
|---|---|---|
| Cell type | Diameter mm |
Height mm |
| AAA | 10.5 | 44.5 |
| AA | 14.5 | 50.5 |
| C | 26.2 | 50.0 |
| D | 34.2 | 61.5 |
| PP3 / 6LR61 | 26.5 x 17.5 | 48.5 |

Note: This form of alkaline battery consists of six cells connected in series within the overall battery package to give the required 9 volt output
How alkaline manganese dioxide cells work
As the name indicates, alkaline manganese dioxide battery cells are based around the use of an alkaline electrolyte and an electrode made from manganese dioxide.
However there is a lot more to these cells than just these components.
Before looking at the actual chemical reactions and how an alkaline manganese dioxide cell works, it is first necessary to look at the structure of this alkaline battery cell.
In terms of its construction, the alkaline cell is the reverse of the older zinc carbon cells, on that the zinc anode is in the centre and the manganese dioxide is the outer active later of the battery cell.
The reason for this is that it gives much better diffusion required for the cell operation and hence it provides a lower electrical internal resistance.
The cell consists of a number of different elements:
• Cathode: The cathode of the alkaline battery cell is made from manganese dioxide. This needs to be produced chemically to obtain sufficient purity (better than about 92%).
The manganese dioxide is then mixed graphite to increase the conductivity as well as some binding agents.
• Anode: Again the anode must be pure to give the correct performance. It is made from zinc (99.8 to 99.9% pure).
Normally the anode is powdered - this is achieved in the manufacturing process for the zinc. The powder is then used for the anode either as a gell , or it can be used as a porous anode.
• Separator: The separator has an important function within any battery or cell and in this case the alkaline cell. As the name indicates, it serves to separate or keep the anode and cathode contacts apart, while also allowing the conduction of the charged ions through the electrolyte.
• Electrolyte: The name of the cell indicates that the electrolyte for the alkaline cell is an alkali. It is normally either potassium or sodium hydroxide. This is contained within the separator.
• Casing: The case of the alkaline battery cell is also important. It forms an integral part of the battery, holding everything in place and providing one of the contacts.
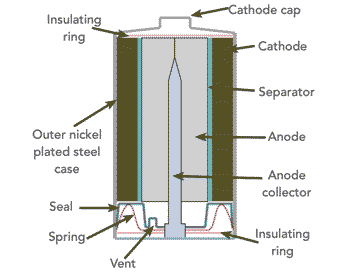
In terms of the actual construction, the overall cell is held within a nickel plated steel casing which provides the mechanical integrity of the overall alkaline cell.
The manganese dioxide power of the cathode is is under pressure against the nickel plated steel container and this provides good contact for the electrical conduction. This makes the steel container what is termed the cathode collector as it enables the current to flow to the outside electrical or electronic circuit. It becomes the positive terminal.
The centre of the alkaline manganese battery cell contains the zinc anode. This is in the form of a zinc power and it often has a gelling agent to help with the porosity of this electrode.
There is an anode current collector which consists of a "nail" like item is placed in the centre of the anode and extends out to the external anode cap to give the external connection.
Between the anode and cathode there is a separator which isolates the two electrodes. It is highly absorbent and it allows the transfer of ions through it.
In addition to this, the intentionally porous nature of the anode and cathode materials enables them to become thoroughly saturated with the alkaline electrolyte solution. Having such porous electrodes vastly increases the surface area of the electrodes and enables the cells to provide a very high level of current.
The overall cell is sealed to prevent loss of the electrolyte which would not only impair the operation of the cell, but it would also cause corrosive damage any equipment using the cell. However there is a vent on the cell to relieve any excess pressure from gas created if the cell is short circuited or disposed in a fire, etc.
The overall chemical reaction for the alkaline cell can be shown to be:
The alkaline manganese dioxide battery cell now holds a dominant position in the marketplace for primary or non-rechargeable cells. It has a much higher charge capacity than the older zinc carbon cells and with modern manufacturing techniques and the technology used for its manufacture, it is much less prone to leaking, although this is still possible.
The alkaline cells are widely used for small electrical and electronic items ranging from toys to torches and many other items. They are cheaper than lithium ion rechargeable cells or batteries, and have a 3 volt output rather than 1.5 per cell. The cost may sometimes be the deciding factor that means that alkaline cells rather than lithium ion ones are used for a given electronic circuit design.
 Written by Ian Poole .
Written by Ian Poole .
Experienced electronics engineer and author.
More Electronic Components:
Batteries
Capacitors
Connectors
ADC
DAC
Diodes
FET
Inductors
Memory types
Phototransistor
Quartz crystals
Relays
Resistors
RF connectors
Switches
Surface mount technology
Thyristor
Transformers
Transistor
Unijunction
Valves / Tubes
Return to Components menu . . .